Disorders of the basal ganglia produce a group of diseases called movement disorders. There is either a decrease in movement as in parkinsonism or an increase in movements as in tremors, dystonia, chorea, athetosis, ballism, myoclonus or tics. In this session, we will discuss the anatomy and physiology of the basal ganglia. We will learn how to approach a patient with parkinsonism, one of the most common movement disorders.
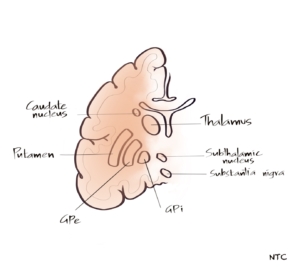
There is no consensus as to which all structures to be included in the basal ganglia. In this discussion, we will keep it simple and basic. The basal ganglia consist of subcortical nuclei: the caudate nucleus, putamen, globus pallidus externa, globus pallidus interna, subthalamic nucleus, and substantia nigra. The caudate and putamen together are called the striatum. The putamen and globus pallidum together are called the lentiform nucleus. The basal ganglia and their connections are often referred to as the extrapyramidal system. The anatomist often disagrees with that because there are other important non-pyramidal motor systems that are not connected to the basal ganglia like the rubrospinal, vestibulospinal, olivospinal, and reticulospinal tracts and the cerebellum. The basal ganglia control the posture and movements. According to Marsden, basal ganglia is responsible for executing learned motor acts like walking. Much of its function arises through the modulation of the pyramidal system. It does not directly produce voluntary movement but is closely integrated with the motor system to modulate and regulate the motor activity carried out through the pyramidal system. The basal ganglia have limbic connections involved with the emotional aspects of the movement. It has links with the oculomotor system. Basal ganglia play a role in cognition as well. Now let us see how basal ganglia modulate the motor activity of the pyramidal tract.
Fig 1- Anatomy of basal ganglia
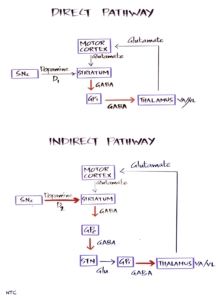
Basal ganglia have significant internuclear connections. Five different circuits through the basal ganglia are proposed. The motor, oculomotor, dorsolateral prefrontal, orbitofrontal and mesial frontal. The latter three are associated with cognitive and emotional functions. The basal ganglia control the movements by influencing the primary and supplementary motor cortex. This is done through the Cortico-striato-pallido-thalmo-cortical pathway. The thalamus stimulates the motor cortex to produce movements. But it is under the inhibitory control of GPi. The cortex stimulates the striatum, and the striatum fine-tunes movements through its control over GPi. This is done through two pathways. The direct pathway directly inhibits GPi, so that the inhibition on the thalamus is lost, favouring further movement. The indirect pathway from the striatum to GPi through GPe and STN stimulates GPi so that the thalamus remains inhibited and movements are decreased. So, we can remember it as the indirect pathway inhibits movement and the direct pathway increases movement. In the direct pathway, the striatum is directly connected to Gpi. The striatum is connected to Gpi through the GPe and STN in the indirect pathway, which is the only difference between the direct and indirect pathways. The substantia nigra SNc has a significant connection with the striatum. The projections from substantia nigra facilitate activity in the direct pathway, mediated via D1 dopamine receptors, and inhibit activity in the indirect pathway via D2 dopamine receptors resulting in increased movements. In PD, there are decreased dopaminergic neurons in SN, resulting in decreased movements. Similarly, there is an increased loss of neurons in the indirect pathway in Huntington’s disease, resulting in increased movements. This is an oversimplification of basal ganglia function, but it will help to understand the basic functional physiology of basal ganglia. In this session, we will be confining our discussion to basal ganglia disorder with decreased movements, namely parkinsonism.
Fig 2- The direct and indirect pathway of basal ganglia
Parkinsonism is a syndrome of bradykinesia with rigidity or tremor. The disease of the basal ganglia causes it. Parkinson’s disease is responsible for about 75% of parkinsonism cases. It is the second common neurodegenerative disease after Alzheimer’s disease. Let us discuss a case. A 65-year-old retired army officer presented to the outpatient department with the slowness of activities of 4 months duration. The first symptom noticed was when he and his wife used to go for morning walks, he started to lag behind his wife. Previously he used to walk faster than her. He began to take more time to eat and bathe. He noticed tremors in his right hand at rest. On examination, he had decreased arm swing on the right side while walking. He had cogwheel rigidity on his right upper limb, and there was bradykinesia and rest tremor on his right hand. His brain imaging and lab investigations were normal. A provisional diagnosis of Idiopathic Parkinson’s disease was made, and the patient was started on low dose levodopa with symptomatic improvement.
This is a prototype PD case. The most important feature of Idiopathic PD is asymmetry. In most cases, it starts on one side and involves the other side as the disease progresses. The cardinal features of PD include rest tremor, rigidity, bradykinesia, and postural instability. The pathological hallmark features of PD are degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNc) and reduced striatal dopamine. The result is a decreased activity in the direct pathway mediated by D1 receptors and increased activity in the indirect pathway mediated by D2 receptors leading to decreased movements. Patients initially respond very well to levodopa, another critical feature of Idiopathic PD.
Parkinsonism can occur secondary to drugs, toxins, infection, trauma, liver disease and tumours, which affect the basal ganglia. Secondary parkinsonism is the next common cause of parkinsonism after Idiopathic Parkinson’s disease, accounting for about 15-20% of cases. Let us discuss a case. A 55-year-old school teacher came with the slowness of activities of 3 months duration. On examination, he had bilateral symmetrical cogwheel rigidity and bradykinesia. The strictly symmetrical parkinsonism was a red flag for Idiopathic Parkinson’s disease diagnosis. On retaking history, it was found that he was taking over the counter flunarizine tablet for the last year. He was advised flunarizine for two weeks for his migraine by his family doctor, but he continued the medication for a year. Flunarizine is well known to produce secondary parkinsonism due to its D2 blocking property. The drug was stopped, and the patient was started on central anticholinergic trihexyphenidyl. The patient completely recovered in a couple of months.
It is vital to rule out secondary causes in all cases of parkinsonism, as illustrated by this case. The other drugs commonly associated with parkinsonism include antipsychotic medications, metoclopramide, reserpine, alpha methyldopa, tetrabenazine, levosulpiride, lithium and amiodarone. It is essential to take a detailed drug history, including indigenous medicines in all patients. The toxins producing secondary parkinsonism include cyanide, carbon monoxide, MPTP, manganese and methanol. These toxins can damage basal ganglia and produce parkinsonism. Certain infections like Japanese encephalitis have a predilection for basal ganglia and can produce secondary parkinsonism. People exposed to recurrent head trauma as in contact sports like boxing can develop secondary parkinsonism and cognitive decline called dementia pugilistica. Similarly, stroke and tumours in the basal ganglia region can produce parkinsonism. Metabolic disorders like hypoparathyroidism and liver disease can also cause basal ganglia dysfunction.
After Idiopathic PD and secondary parkinsonism, the next common cause of parkinsonism is parkinsonian plus syndromes or atypical parkinsonism. They are neurodegenerative disorders with more widespread pathology than PD. These include Progressive supranuclear palsy[PSP], Corticobasalganglia degeneration[CBD] and Multisystem atrophy[MSA]
A 65-year-old policeman presented with the slowness of activities and recurrent falls of 6 months duration. He did not have a history of tremors. There was emotional incontinence. He had trunkal rigidity more than axial with vertical gaze palsy on eye movements examination. MRI brain showed midbrain atrophy consistent with the clinical diagnosis of PSP. PSP is a tauopathy characterised by akineto-rigid syndrome, early falls and vertical gaze palsy. The patient can also have bipyramidal signs, emotional incontinence and cognitive decline. The midbrain atrophy is seen in MRI as the hummingbird sign in the sagittal or morning glory signs on the axial cuts. The response to levodopa is poor.
A 58-year-old housewife presented with the slowness of activity of 6 months duration associated with abnormal, painful posturing of her right upper limb. On examination, she had jerky movements of her right upper limb, which increased on touching the limb. The painful dystonia and stimulus sensitive myoclonus made the right upper limb almost functionless, and she was using her left upper limb for her day-to-day activities. The bradykinesia and rigidity were more on the right side than the left. The detailed lobe function tests showed a significant cognitive decline. MRI brain showed asymmetric atrophy of the left parieto temporal region consistent with the clinical diagnosis of Corticobasal syndrome. Corticobasal degeneration [CBD] is a parkinsonian plus syndrome characterised by asymmetrical parkinsonism with early apraxia, dystonia, cognitive involvement and other cortical features like loss of cortical sensory loss. It is a tauopathy like PSP and Frontotemporal dementia. Some patients may show alien limb phenomenon where a limb will act without the patient’s awareness. In an extreme case, it will obstruct the work done by the opposite limb, called inter manual conflict. Apraxia, an inability to do learned motor activities such as brushing teeth, is seen early in the disease. As the disease progress, it will be difficult to test as the limb become functionless due to the severe rigidity and painful dystonia. The patient will have other cortical features like cortical sensory loss, cortical myoclonus, aphasia and dementia. Like in other atypical parkinsonism, the response to levodopa is poor.
A 56-year-old banker presented with bradykinesia, swaying while walking, and urinary incontinence of 6 months duration. He used to have violent enactment of dreams at night for the last several years, and for the previous three months, he had stridor in sleep. On examination, he had significant postural hypotension of 30mm systolic BP fall on standing. He had mild parkinsonian features in the form of rigidity and bradykinesia and significant cerebellar signs. He was started on levodopa from outside for Parkinsonian symptoms when he developed postural syncope. Given the significant autonomic, cerebellar and extrapyramidal involvement, a possibility of Multisystem atrophy MSA-C was considered. MRI brain showed the classic hot cross bun sign in the pons consistent with the clinical diagnosis of MSA. This is a prototype MSA case. Unlike PSP, CBD and FTD, MSA is a synucleinopathy. Lewy body dementia is another synucleinopathy where dementia with parkinsonism occurs. As the name suggests, multiple systems, including extrapyramidal, cerebellar, autonomic and pyramidal tracts, are involved in MSA. Dysautonomic features, including postural hypotension, urinary incontinence or retention, erectile dysfunction and constipation, are almost universally seen. If parkinsonism is prominent, it is called MSA P, and if cerebellar signs are prominent, MSA-C. Inspiratory stridor can occur, while cognitive impairment is not usually seen in MSA. REM sleep behavioural disorders are common as other synucleinopathies and occur much before other symptoms. Like other atypical parkinsonism, the response to levodopa is poor and can often worsen postural hypotension. It’s important to recognise the possibility of atypical parkinsonism and secondary parkinsonism when patients present with parkinsonism. Some of the red flags include
-
- Early falls
- Diplopia and vertical gaze restriction
- Early cognitive decline
- Early significant autonomic symptoms
- Apraxia, aphasia and other cortical features
- Poor response to levodopa
- Exposure to drugs and toxins
- Babinsky sign and other pyramidal signs
- Cerebellar signs
- Early-onset and presence of liver disease
Idiopathic PD, secondary parkinsonism and atypical parkinsonism together form more than 95% of all parkinsonism cases. The rest includes neurodegenerative disorders, with parkinsonism as one of its symptoms. These include Wilson’s disease, SCA 3, Huntington’s disease, NBIA, Prion disease, DOPA responsive dystonia etc. A good clinical history and detailed clinical examination will help to differentiate the various causes of parkinsonism.

Nice forum